Daniel Wozniak
Professor of Microbial Infection & Immunity and Microbiology
704 Biomedical Research Tower
Areas of Expertise
- Bacterial pathogenesis
- Gene regulation
Education
- Ph.D. Ohio State University, 1989
- Postdoc, University of Tennessee, 1989-1992
- Faculty, Wake Forest University, 1992-2008
Research Interests
Dr. Wozniak’s research activities are focused on the pathogenesis of several bacteria that cause chronic, devastating infections in humans. In chronic airway infections and wounds. Pseudomonas aeruginosa, Staphylococcus aureus, and Acinetobacter are the most common nosocomial pathogens isolated and are consistently associated with high mortality rates. Resources spent treating such infections in the U.S. are estimated at ~ $25 billion annually. These infections are extremely difficult to control since the bacteria exhibit a biofilm-mode of growth rendering them resistant to antimicrobials and phagocytic cells. Collectively, our research activities are focused on:
Understanding the coordinate regulation of the virulence factors that contribute to P. aeruginosa pathogenesis in CF.
P. aeruginosa has evolved a complex series of mechanisms for regulation of the virulence factors that contribute to pathogenesis. These regulation pathways have been the focus of intense research as potential targets for anti-microbial compounds. Our current emphasis deals with the regulation of expression of the polysaccharide alginate, a capsular polysaccharide virulence factor that confers a selective advantage for P. aeruginosa in CF.
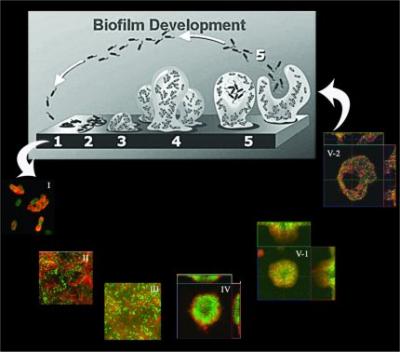
Understanding the process of biofilm formation.
Biofilms, which are defined as communities of microorganisms that are attached to a surface, play a critical role in infectious diseases. Because of their innate resistance to antibiotics, phagocytic cells, and other biocides, biofilms are difficult, if not impossible, to eradicate. This represents a critically important challenge for anti-infective programs in the pharmaceutical industry. Ourcurrent work has focused on the role of Psl and Pel polysaccharides, as well as type IV mediated twitching motility, in P. aeruginosa biofilm development.
Experimental therapeutics for use in patients infected with P. aeruginosa.
The matrix contributes considerably to the highly resistant nature of microbial biofilms. In collaborative studies, we are developing novel vaccines and drugs that could inhibit growth and/or formation of the biofilm matrix. Such agents may be of significant therapeutic value in patients colonized with P. aeruginosa.
Defining pathoadaptive processes and evolution of P. aeruginosa during infection.
Patients with CF become colonized with multiple pathogens. During the course of infection, P. aeruginosa undergoes a phenotypic conversion to a mucoid phenotype due to the overproduction of alginate.Mucoid conversion results from mutations occurring in genes that regulate alginate synthesis. Since P. aeruginosa mucoid conversion is associated with increased patient morbidity and mortality, we are investigating both the molecular mechanisms and the host-factors that may promote mucoid conversion.
Development and use of a chronic wound infection model to evaluate molecular mechanisms of persistence and potential anti-biofilm therapeutic strategies.
To develop novel strategies that combat biofilm infections, a greater understanding of their properties needs to be developed. Importantly, special focus needs to be given to the interaction of the immune system and biofilms, the development of models that closely mimic human disease, and the translation of this information to patient care. In collaboration with Dr. Chandan Sen at OSU, our groups have developed a preclinical model of chronic infection that limits wound healing and correlates with biofilm formation. The deployment of this model as well as the direct comparison to human tissue samples derived from burn wounds will greatly accelerate the study of biofilm infections due to the ability to translate our findings directly to humans. The development of this model of infection that faithfully reproduces what is observed in human disease is critical for testing future preclinical treatment modalities.
Memberships
Member, CMIB
Member, DID
Member, IBGP
Member, PHPID
Relevant Publications
Ma L, Wang S, Parsek MR & Wozniak DJ. (2013). A spider-web mechanism of type IV pili-mediated migration to build a fiber-like Psl polysaccharide matrix in Pseudomonas aeruginosa biofilms. Env. Microbiol. doi: 10.1111/1462-2920.12095
Jones CJ, Ryder CR, Mann EE & Wozniak DJ. (2013). AmrZ modulates Pseudomonas aeruginosa biofilm architecture by directly repressing transcription of the psl operon. J. Bacteriol. 195:1637-1644.
Irie Y, Borlee BR, O’Connor JR, Hill PJ, Harwood CS, Wozniak DJ & Parsek MR. (2012). Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. U.S.A. 109:20632-20636.
Zegans ME, Wozniak DJ, Griffin E, Toutain-Kidd CM, Hammond JH, Garfoot A & Lam JS. (2012). Pseudomonas aeruginosa exopolysaccharide Psl promotes resistance to the biofilm inhibitor polysorbate 80. Antimicrob Agents Chem. 56: 4112-4122.
Ma L, Wang J, Wang S, Ryder C, Anderson CM, Lam JS, Parsek MR & Wozniak DJ. (2012). Synthesis of multiple Pseudomonas aeruginosa biofilm matrix expolysaccharides is post-transcriptionally regulated. Env. Microbiol. 14(8):1995-2005.
Pryor EE, Waligora EA, Xu B, Dellos-Nolan S, Wozniak DJ & Hollis T. (2012). The transcription factor AmrZ uses multiple DNA binding modes to recognize activator and repressor sequences of Pseudomonas aeruginosa virulence genes. PLoS Pathogens. 8(4): e1002648.
Ma L, Wang S, Wang D, Parsek MR & Wozniak DJ. (2012). The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Micro. 65(2):377-380.
Mishra M, Byrd MS, Sergeant S, Azad A, Parsek MR, McPhail L, Schlesinger LS & Wozniak DJ. (2012). Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cellular Micro. 14:95-106.
Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, Ryder C, Howell PL, Wozniak DJ & Parsek MR. (2012). The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol. 4(8):1913-1928.
Mann E & Wozniak DJ. (2011). Pseudomonas biofilm matrix composition and function. FEMS Microbiol. Rev: 36(4): 893-916
Byrd MS, Pang B, Hong W, Waligora EA, Juneau RA, Armbruster CE, Weimer KED, Murrah K, Mann EE, Lu H, Sprinkle A, Kock ND, Parsek MR, Wozniak DJ & Swords WE. (2011). Direct evaluation of Pseudomonas aeruginosa biofilm mediators in a chronic infection model. Infect. Immun. 79:3087-3095.
Sanders LH, Devadoss B, Raja GV, O’Connor J, Su S, Wozniak DJ, Hassett DJ, Berdis AJ & Sutton MD. (2011). Epistatic roles for Pseudomonas aeruginosa MutS and DinB (DNA Pol IV) in coping with reactive oxygen species-induced DNA damage. PLoS One. 2011;6(4):e18824.
Colvin K, Gordon V, Murakami K, Borlee BR, Wozniak DJ, Wong G & Parsek MR. (2011). The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathogens, 7(1): e10001264. Doi:10.1371/journal.ppat.10001264.
