John Gunn
Professor of Pediatrics and Microbiology and Principal Investigator, Nationwide Children's Hospital
794 Biomedical Research Tower
Areas of Expertise
- Salmonella and Francisella host-pathogen interactions
Education
- Ph.D. University of Maryland, 1993
- Postdoc, Harvard Medical School, 1993-1995
- Postdoc, University of Washington, 1995-1997
Research Interests
Salmonella/Francisella pathogenesis and host cell interactions
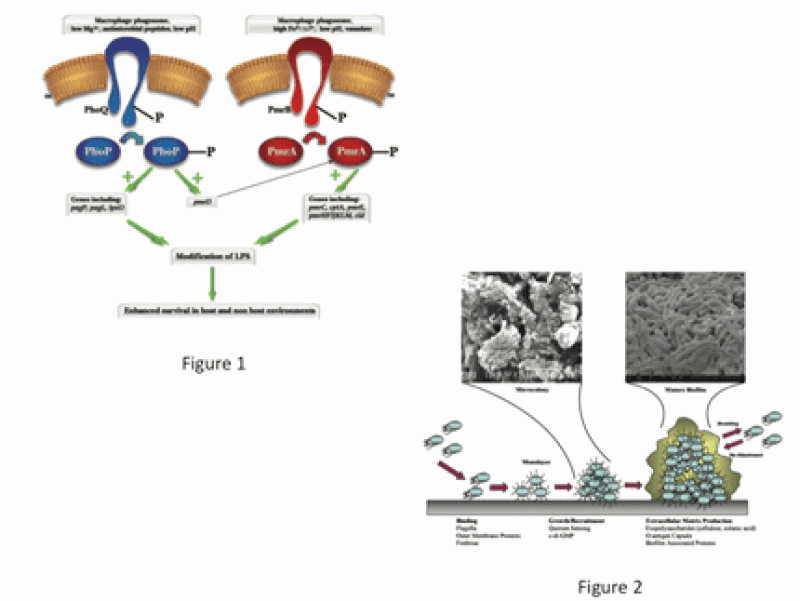
My laboratory studies the pathogenic mechanisms of acute and chronic infections caused by the facultative intracellular pathogensSalmonella Typhi/Typhimurium and Francisella tularensis (Ft). We focus on virulence gene regulation, intramacrophage survival, resistance to innate immune killing, biofilm formation and the development of therapeutics/vaccines.
We are interested in the molecular mechanisms used by Salmonella spp. to survive harsh conditions it encounters within the human host, particularly mucosal innate immune defenses, the macrophage phagosome and the gallbladder (GB). Present within host phagocytes and at mucosal surfaces are a potent group of cytotoxic agents, antimicrobial peptides (AP). The PhoP-PhoQ and PmrA-PmrB two-component regulatory systems are activated in vivo and are necessary for resistance to AP, which involves modifications of the LPS that decrease peptide binding and bacterial cell entry (Fig. 1). We are primarily focused on studies of the PmrAB regulon, including the identification and characterization of PmrAB-regulated genes necessary for AP resistance and LPS modification, and the activation of/role of PmrAB-mediated LPS modifications in vivo during Salmonella infection.
Approximately 3-5% of individuals infected with S. Typhi become chronic carriers with the GB as the primary site of persistence. S. Typhi is a human-restricted pathogen, therefore asymptomatic carriers represent a critical reservoir for further spread of disease. We have demonstrated that gallstones (GSs) aid in the development and maintenance of GB carriage in a mouse model and in humans, serving as a substrate to which salmonellae attach and form a protective biofilm (Fig. 2). However, the molecular basis of chronic carriage of Salmonella in the GB, both from the host and bacterial perspectives, is poorly understood. Our goal is to better understand the environment that allows for asymptomatic chronic carriage and to develop therapies to reverse/prevent it.
Following infection, Ft alters intracellular trafficking within cells, escapes from the phagosome, replicates to high levels in the host cell cytosol, induces apoptosis and autophagy, and actively induces immunosuppression within the infected cell. Studies by our group and others indicate that these events are likely to be coordinated by transcriptional regulators. Genomic analysis of various Ft subspecies indicates that these organisms encode a paucity of transcriptional regulators (N=~35), yet they survive and replicate in numerous environmental niches as well as in animals, insects, arthropods, and protozoa. Given this dichotomy, and the demonstrated importance of several transcription factors inFt growth and virulence, we speculate that the limited number of protein transcriptional regulators and newly identified small RNAs encoded in the Ft genome must play key roles in the physiology and pathogenesis of Ft within host cells. Identified regulators may represent important new targets for the development of vaccines or therapeutics against Ft infection. Our recent focus has involved an orphan response regulator gene (PmrA) that interacts with other transcriptional regulators to regulate the Francisella pathogenicity island (FPI) genes. We have also identified sRNAs that play a significant role in FPI regulation and virulence.
We continue to investigate the biology and role in virulence of the four Francisella acid phosphatases. These gene products are involved in macrophage vacuolar escape, trafficking within the macrophage, and inhibit the respiratory burst. Our recent data show that at least one of these acid phosphatases is secreted and translocated out of the phagosome by an unknown secretion system. We seek to understand the mechanism of secretion and the host cell targets for these acid phosphatases.
Memberships
Vice Director, CMIB
Member, DID
Member, DHLRI
Member, OSBP
Member, PHPID
Recent Publications
- Gunn JS, Marshall J, Baker S, Dongol S, Charles RC & Ryan ET. (2014).Salmonella chronic carriage: epidemiology, diagnosis and gallbladder persistence. Trends in Micro. Accepted.
- Marshall JM, Flechtner AK, La Perle KM & Gunn JS. (2014). Visualization of extracellular matrix components within sectioned Salmonella biofilms on the surface of human gallstones. PLoS One. Feb 14;9(2):e89243.PMID: 24551241; PMCID: PMC3925243.
-
Mohapatra NP, Soni S, Murugesan RV, Strandberg KL & Gunn JS. (2013). Type A Francisella tularensis acid phosphatases contribute to pathogenesis. PLoS ONE 8(2): e56834. doi:10.1371/journal.pone.0056834 PMCID: PMC3574111.
-
Gonzalez-Escobedo G & Gunn JS. (2013). The Gallbladder Epithelium as a Niche for Chronic Salmonella Carriage. Infect Immun. 2013 Aug;81(8):2920-30. doi:10.1128/IAI.00258-13. Epub 2013 Jun 3. PubMed PMID: 23732169; PubMed Central PMCID: PMC3719562.
-
Gonzalez-Escobedo G & Gunn JS. (2013). Identification of Salmonella factors regulated during biofilm formation on cholesterol (gallstone) surfaces. Infect Immun. 2013 Jul 29. PubMed PMID: 23897604.
-
Gonzalez-Escobedo G, La Perle KM & Gunn JS. (2013). Histopathological analysis of Salmonella chronic carriage in the hepatopancreatobiliary system. PLoS One. 2013 Dec 12;8(12)
-
Collier MA, Gallovic MD, Peine KJ, Gunn JS, Schelesinger LS, Ainslie KM. (2013). Delivery of host cell-directed therapeutics for intracellular pathogen clearance. Expert Review of Anti Infective Therapy. Expert Rev Anti Infect Ther. Nov; 11(11): 1225-35. PMID: 24134600
-
Strandberg KL, Richards SM & Gunn JS. (2012) Cathelicidin Antimicrobial Peptide Expression Is Not Induced or Required for Bacterial Clearance during Salmonella enterica Infection of Human Monocyte-Derived Macrophages. Infect Immun. 80(11):3930-8.
-
Dai, S., Mohapatra MP, Schlesinger LS & Gunn JS. (2012). The acid phosphatase AcpA is secreted in vitro and in macrophages by Francisella spp. Infect. Immun., 80(3):1088-97 PMID: 22184418
-
Gonzalez-Escobedo G, Marshall JM, & Gunn JS. (2010) Chronic and acute infection of the gallbladder by Salmonella Typhi: understanding the carrier state. Nature Reviews Microbiology, Nov. PMID: 21113180
-
Soni S, Ernst RK,Muszynski A, Mohapatra NP, Perry MB, Vinogradov E, Carlson R, & Gunn JS. (2010) Francisella tularensis blue-gray phase variation involves structural modifications of lipopolysaccharide O-antigen, core and lipid A and affects intramacrophage survival and vaccine efficacy. Front. Microbio. 1:129.
-
Mohapatra NP, Soni S, Rajaram MV, Dang PM, Reilly TJ, El-Benna J, Clay CD,Schlesinger LS & Gunn JS. (2010) Francisella acid phosphatases inactivate the NADPH oxidase in human phagocytes. J Immunol. 184, 5141-50.
-
Bell BL, Mohapatra NP & Gunn JS. (2010) Regulation of virulence gene transcripts by the Francisella novicida orphan response regulator PmrA: role of phosphorylation and evidence of MglA/SspA interaction. Infect Immun. 78, 2189-98. Selected for "Spotlight" section.
-
Crawford RW, Rosales-Reyes R, Ramírez-Aguilar Mde L, Chapa-Azuela O, Alpuche-Aranda C & Gunn JS. (2010) Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A. 107, 4353-8. Selected for Press coverage and "This week in PNAS" sections.
-
Crawford RW, Reeve KE & Gunn JS. (2010) Flagellated but not hyperfimbriated Salmonella enterica serovar Typhimurium attach to and form biofilms on cholesterol-coated surfaces. J Bacteriol. 192, 2981-90.
-
Chiu HC, Soni S, Kulp SK, Curry H, Wang D, Gunn JS, Schlesinger LS & Chen CS. (2009) Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J Biomed Sci. 16, 110.
-
Chiu HC, Kulp SK, Soni S, Wang D, Gunn JS, Schlesinger LS & Chen CS. (2009) Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob Agents Chemother. 53, 5236-44.
-
Mohapatra NP, Soni S, Reilly TJ, Liu J, Klose KE & Gunn JS. (2008) The combined deletion of four Francisella acid phosphatases attenuates virulence and macrophage vacuolar escape. Infect Immun. 76, 3690-9.
-
Gavrilin MA, Bouak l , Knatz N, Duncan M, Hall MW, Gunn JS & Wewers MD. (2006) Internalization and phagosome escape required for live Francisella to induce human monocyte IL-1b processing and release. Proc Natl Acad Sci U S A. 103, 141-6.
